


University of Minnesota
Department of Neuroscience
About Our Research
Epilepsy
1 in 26 individuals are at risk of developing epilepsy in their lifetime. Defined by the emergence of spontaneous recurrent seizures, epilepsy develops in a subset of individuals after an injury, such as head trauma, stroke, or central nervous system infection.
Inflammation is a prominent feature of brain injury. We are beginning to understand how inflammation and the immune system may contribute to the risk of developing epilepsy after injury.
Microglia and Calcium Activity
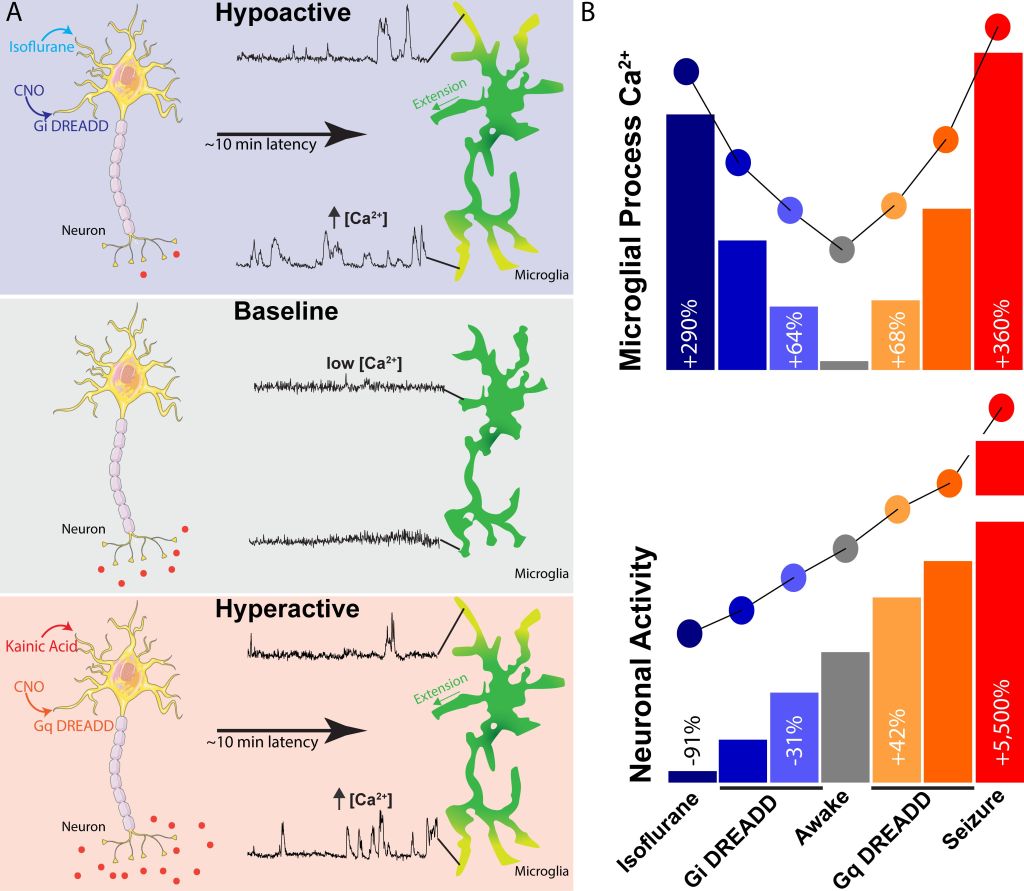
Microglia are the major immune cell found in the central nervous system (CNS). They are known for having very motile processes, which constantly survey local brain tissue. In addition to motility, our lab is particularly interested in microglial calcium activity. Microglia have very rare calcium activity in a naive state, but increase their calcium signaling during periods of physical brain injury, inflammation, and changes in neuronal activity, including seizures. A few years ago, we set out to understand the purpose of calcium signaling when it emerged after injury.
Model for how microglial calcium signaling relates to shifts in neuronal activity (eLife, 2020) Creative Commons License 4.0
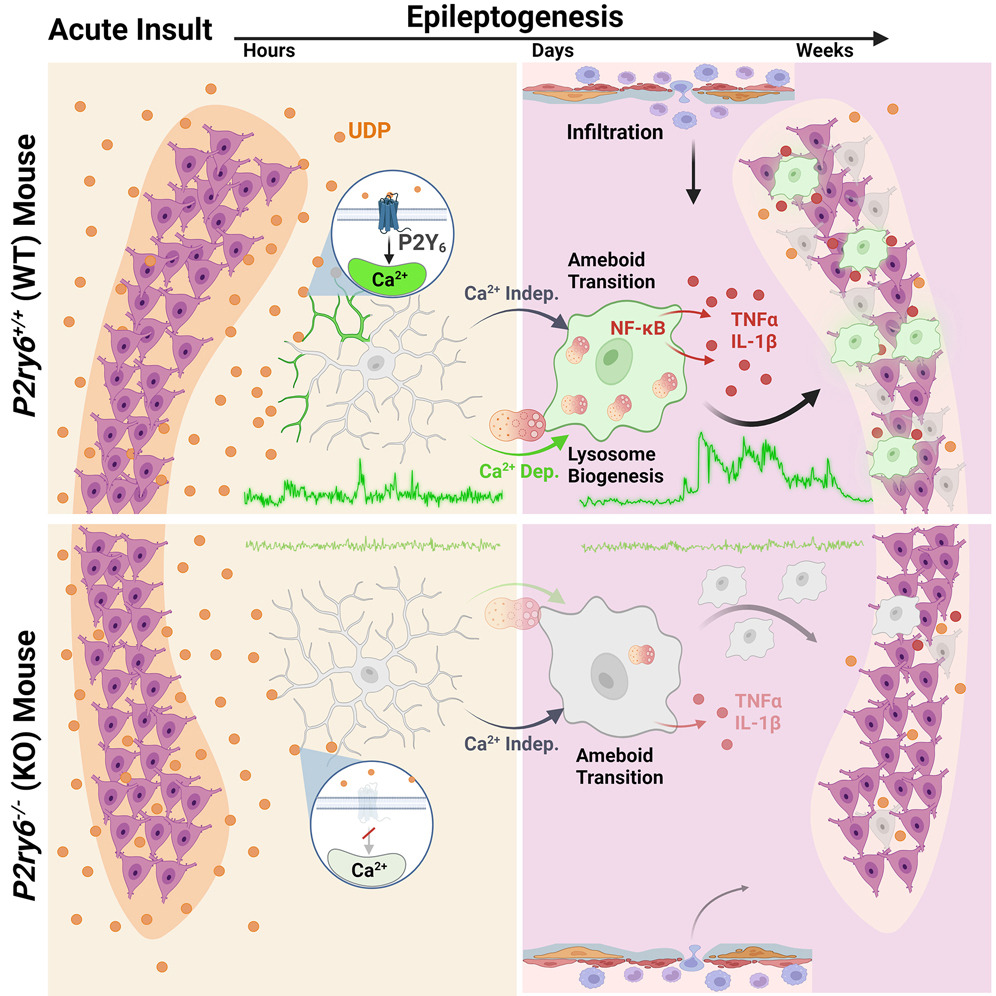
Damage and Inflammation
Microglia rapidly sense damage signals like purines/pyrimidines (ATP/ADP, UTP/UDP) following injury. We use in vivo multiphoton imaging and newly developed purine biosensors to image the dynamics of purine release. UDP release activates a significant amount of calcium signaling in microglia following injury. UDP acts upon the P2Y6 receptor, largely expressed in microglia, to transduce calcium signaling.
Through genetic knockout of the P2Y6 receptor, we found that UDP-P2Y6 calcium signaling mediates multiple aspects of the neuroinflammatory response, including pro-inflammatory cytokine production and phagocytosis/cell loss. We intend to investigate this pathway further for potential therapy development.
Read more about these findings (Neuron, 2024)